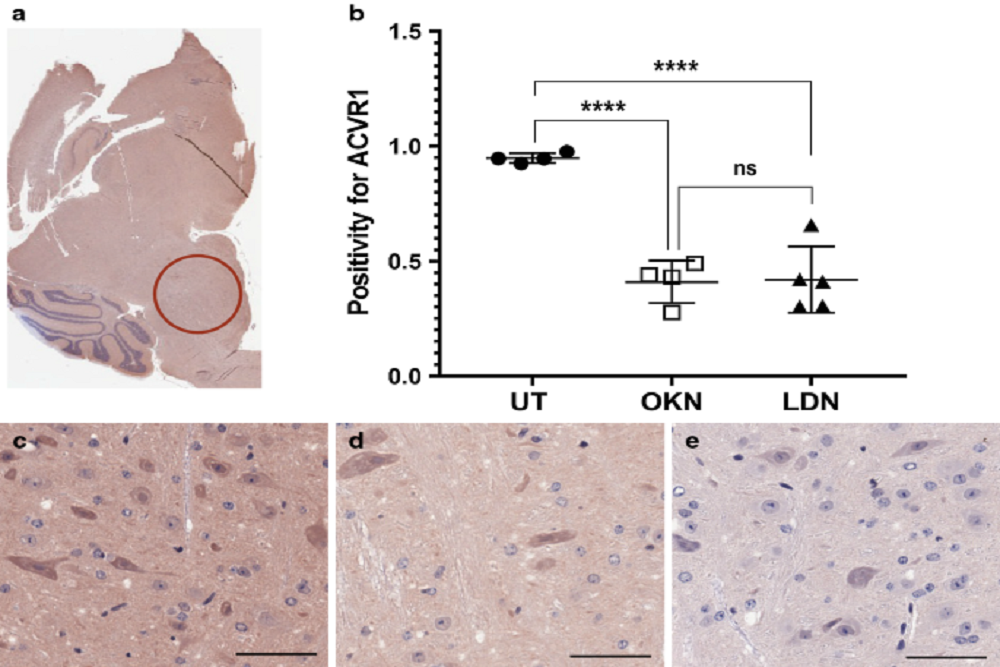
FDA Approves Novadoz Pharmaceuticals Toremifene and Aminocaproic Acid, Bolstering their Continued Growth in Generics
MSN Labs, based in Hyderabad India, recently received FDA approval to market their generic versions of Toremifene 60mg tablets, (AB rated to Kyowa Kirin's Fareston©) & Aminocaproic Acid 500mg tablet, (AB rated Clover's Amicar©) under the Novadoz label.