Astellas Pharma Inc. issued the following announcement on July 16.
Astellas Pharma Inc. (TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., "Astellas") and Seattle Genetics, Inc. (Nasdaq:SGEN) today announced submission of a Biologics License Application for accelerated approval to the U.S. Food and Drug Administration for the investigational agent enfortumab vedotin for the treatment of patients with locally advanced or metastatic urothelial cancer who have received a PD-1/L1 inhibitor and who have received a platinum-containing chemotherapy in the neoadjuvant/adjuvant, locally advanced or metastatic setting.
The submission is based on results from the first cohort of patients in the EV-201 pivotal phase 2 clinical trial that were presented as a late-breaking abstract at the annual meeting of the American Society of Clinical Oncology (ASCO) in June. Enfortumab vedotin is an investigational antibody-drug conjugate (ADC) that targets Nectin-4, a protein that is highly expressed in urothelial cancers.i
"There are limited treatment options for patients with advanced urothelial cancer, and we are encouraged by the results observed in the pivotal trial for enfortumab vedotin," said Andrew Krivoshik, M.D., Ph.D., Senior Vice President and Oncology Therapeutic Area Head at Astellas.
"There is an urgent need for new therapies for patients with advanced urothelial cancer, and we look forward to working with our partner Astellas and the FDA on the review of this application," said Roger Dansey, M.D., Chief Medical Officer at Seattle Genetics.
Based on preliminary results from a phase 1 trial (EV-101), the FDA granted enfortumab vedotin Breakthrough Therapy designation for patients with locally advanced or metastatic urothelial cancer whose disease has progressed during or following checkpoint inhibitor therapy.
A global, randomized phase 3 confirmatory clinical trial (EV-301) is ongoing and is intended to support global registrations. Another ongoing trial, EV-103, is evaluating enfortumab vedotin in earlier lines of treatment for patients with locally advanced or metastatic urothelial cancer, including in combination with pembrolizumab and/or platinum chemotherapy in newly diagnosed patients as well as patients whose cancer progressed from earlier-stage disease.
About the EV-201 Trial
EV-201 is an ongoing single-arm, pivotal phase 2 clinical trial of enfortumab vedotin in patients with locally advanced or metastatic urothelial cancer who have been previously treated with a PD-1/L1 inhibitor and a platinum-containing chemotherapy (cohort 1) and those who have not received a platinum-containing chemotherapy or who are ineligible for cisplatin (cohort 2). In cohort 1, 128 patients were enrolled at multiple centers internationally.ii The primary endpoint is confirmed objective response rate per blinded independent central review. Secondary endpoints include assessments of duration of response, disease control rate, progression-free survival, overall survival, safety and tolerability. EV-201 continues to enroll patients in cohort 2.
More information about enfortumab vedotin clinical trials can be found at clinical trials.gov.
About Urothelial Cancer
Urothelial cancer is the most common type of bladder cancer (90 percent of cases).iii In 2018, more than 82,000 people were diagnosed with bladder cancer in the United States. Globally, approximately 549,000 people were diagnosed with bladder cancer last year, and there were approximately 200,000 deaths worldwide.iv
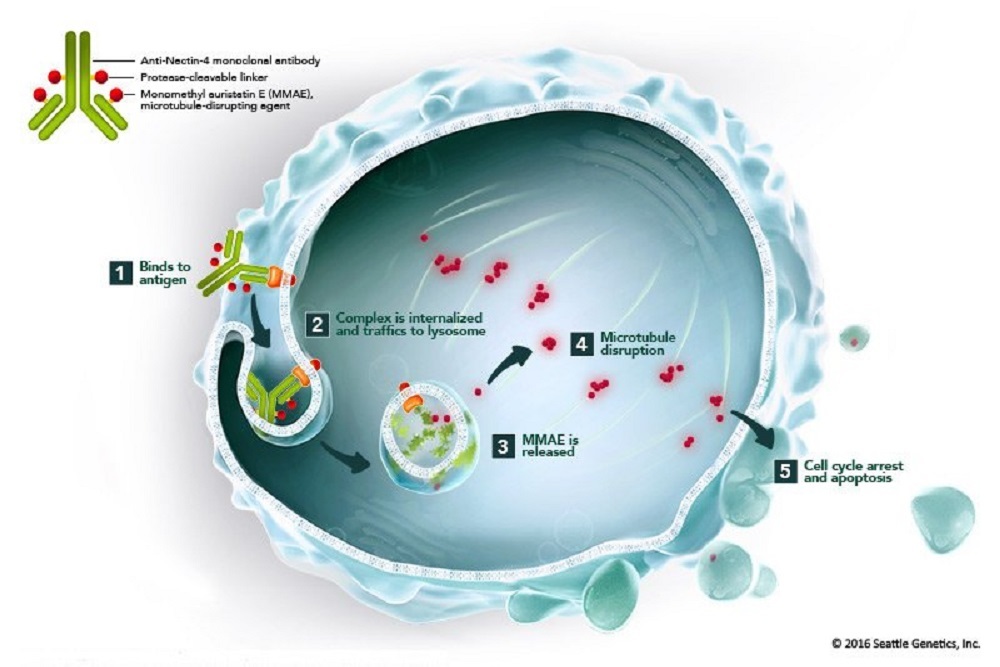
About Enfortumab Vedotin
Enfortumab vedotin is an investigational ADC composed of an anti-Nectin-4 monoclonal antibody attached to a microtubule-disrupting agent, MMAE, using Seattle Genetics' proprietary linker technology. Enfortumab vedotin targets Nectin-4, a cell adhesion molecule that is expressed on many solid tumors, and that has been identified as an ADC target by Astellas.
The safety and efficacy of enfortumab vedotin are under investigation and have not been established. There is no guarantee that the agent will receive regulatory approval or become commercially available for the uses being investigated.
About Seattle Genetics
Seattle Genetics, Inc. is an emerging multi-product, global biotechnology company that develops and commercializes transformative therapies targeting cancer to make a meaningful difference in people's lives. The company is headquartered in Bothell, Washington, and has a European office in Switzerland. For more information on our robust pipeline, visit www.seattlegenetics.com and follow @SeattleGenetics on Twitter.
About Astellas
Astellas Pharma Inc., based in Tokyo, Japan, is a company dedicated to improving the health of people around the world through the provision of innovative and reliable pharmaceutical products. For more information, please visit our website at https://www.astellas.com/en
About the Astellas and Seattle Genetics Collaboration
Seattle Genetics and Astellas are co-developing enfortumab vedotin under a collaboration that was entered into in 2007 and expanded in 2009. Under the collaboration, the companies are sharing costs and profits on a 50:50 basis worldwide.
Original source can be found here.




