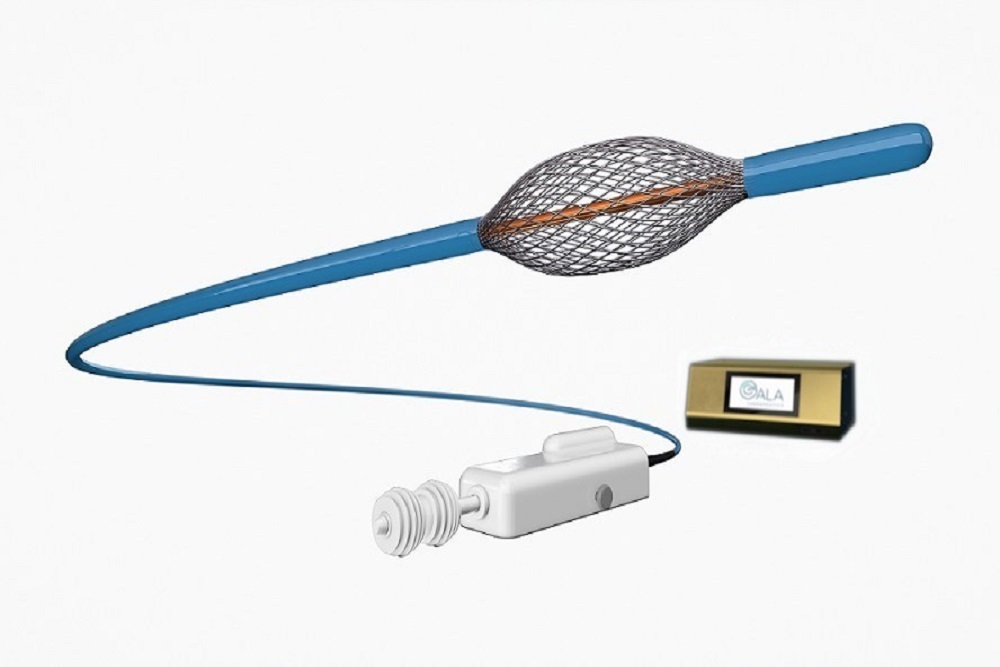
Gala Therapeutics' investigational RheOx Bronchial Rheoplasty system
Gala Therapeutics, Inc. issued the following announcement on Sep. 16.
Gala Therapeutics, Inc. (Gala), a developer of medical devices to treat pulmonary disease, announced the U.S. Food and Drug Administration (FDA) granted Breakthrough Device Designation (FDA) for the RheOxTM Bronchial RheoplastyTM system. RheOx uses a minimally invasive procedure to deliver non-thermal energy to the airways to reduce mucus-producing cells in patients with Chronic Bronchitis.
There are no treatments to address the debilitating symptoms impacting the quality of life in patients with Chronic Bronchitis. We are excited by FDA's decision to designate RheOx as a breakthrough device, underscoring the significant unmet need for these patients," said Jonathan Waldstreicher, MD, CEO of Gala Therapeutics. "We look forward to working with FDA to bring a solution that improves the lives of these patients."
The Breakthrough Device Program is intended to streamline the regulatory pathway for first-of-its-kind medical devices that can provide more effective treatment for patients suffering from life-threatening or irreversibly debilitating diseases to ensure that patients have timely access to vital treatments.
Chronic Bronchitis affects over 9 million people in the U.S., occurring in people with Chronic Obstructive Pulmonary Disease (COPD) and in individuals with normal lung function.1 Patients with Chronic Bronchitis experience persistent cough, excess mucus production, and impaired quality of life with an increased risk of exacerbations and mortality.2 Current treatments are directed at bronchodilation and reduction in inflammation only, without addressing the overproduction of mucus.
About RheOxTM
RheOx is a bronchoscopic system designed to reduce mucus-producing cells in patients with Chronic Bronchitis. The revolutionary technology includes an electrosurgical generator and a single-use catheter that together deliver non-thermal energy to the airways to reduce the number of abnormal mucus-producing cells in the lungs, making way for new normal cells to redevelop. Currently under evaluation in an early feasibility study in the United States, RheOx is limited by federal (or United States) law to investigational use. RheOx received CE certification earlier this year.
About Gala Therapeutics
Gala Therapeutics is a privately held medical device company based in Menlo Park, CA, that is dedicated to developing disease-modifying therapies that improve survival, quality of life, and outcomes for patients with pulmonary diseases. Formed by Apple Tree Partners, a healthcare-focused venture capital firm based in New York, Gala is building a portfolio of technologies to address the needs of interventional pulmonologists, thoracic surgeons, and all physicians who treat pulmonary disease.
Original source can be found here.




