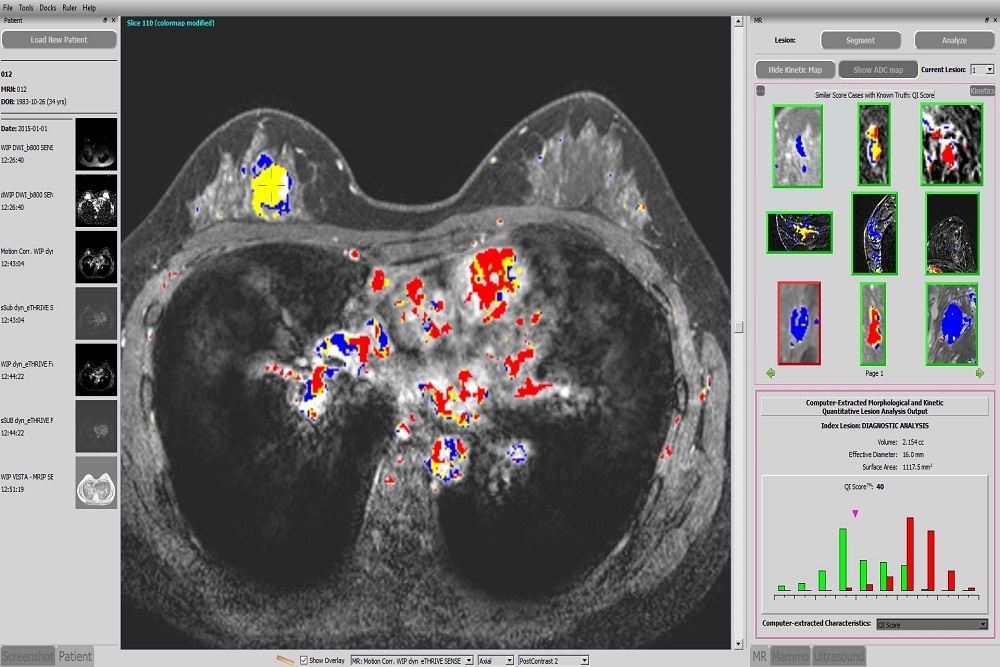
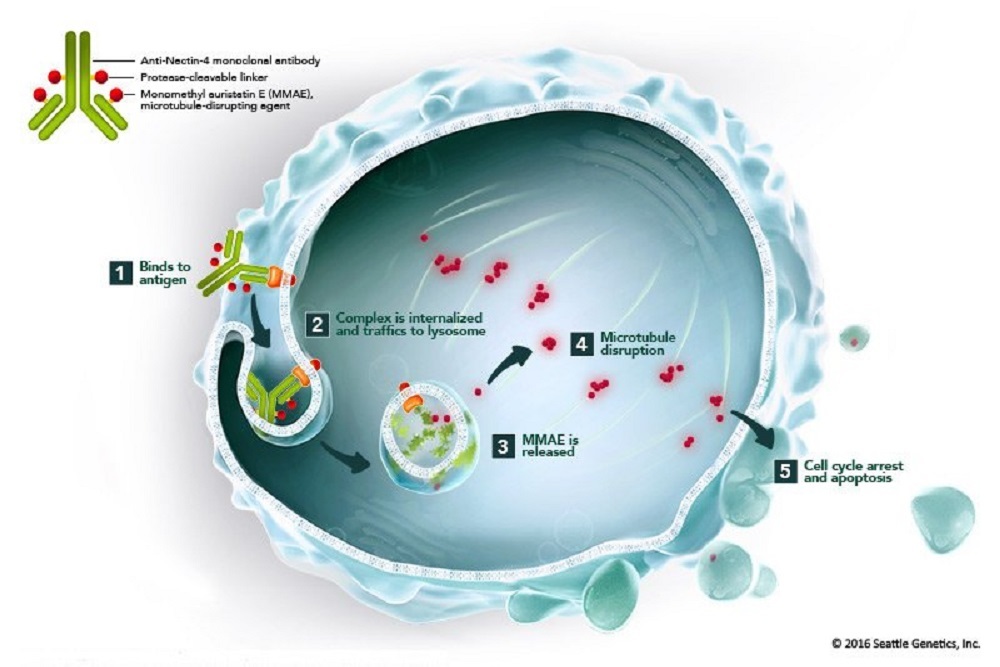
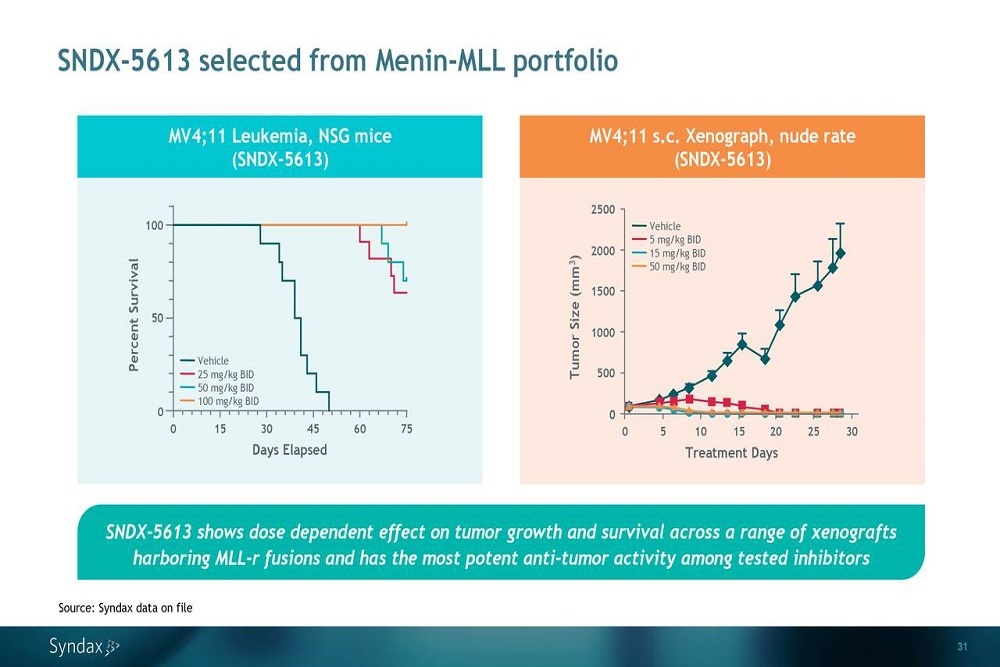
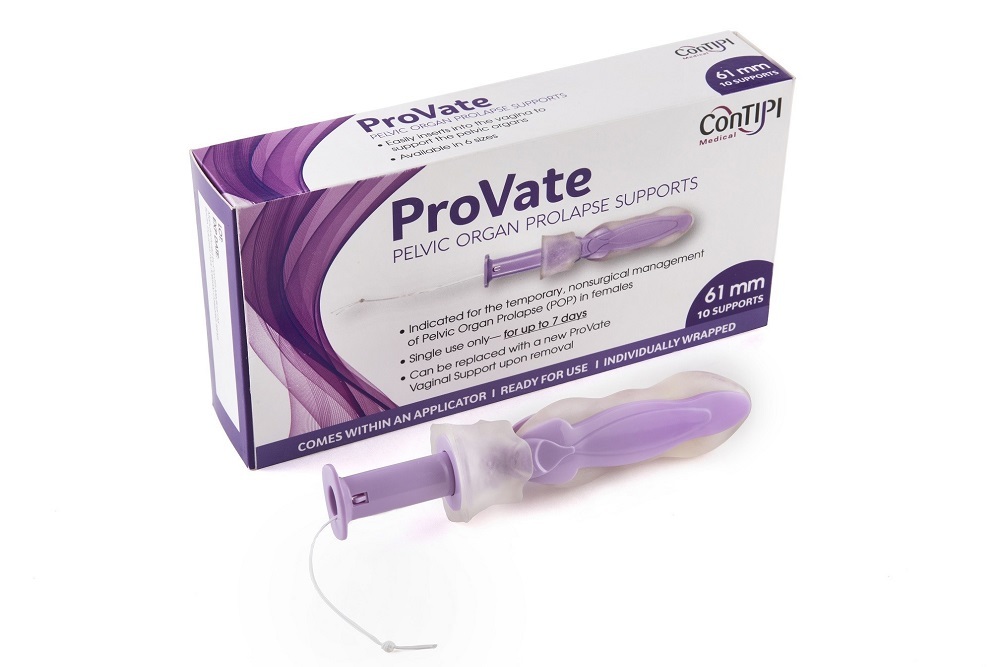
VERTEX PHARMACEUTICALS: Vertex Submits New Drug Application to the U.S. FDA for Triple Combination Regimen of VX-445 (Elexacaftor), Tezacaftor and Ivacaftor in Cystic Fibrosis
Application supported by positive results from two global Phase 3 studies in people with CF ages 12 and older with one F508del mutation and one minimal function mutation and in people with two F508del mutations