BIOTIME, INC.: Announces Issuance of U.S. Patent for Method of Reducing Cavitation in Patients with Acute Spinal Cord Injury
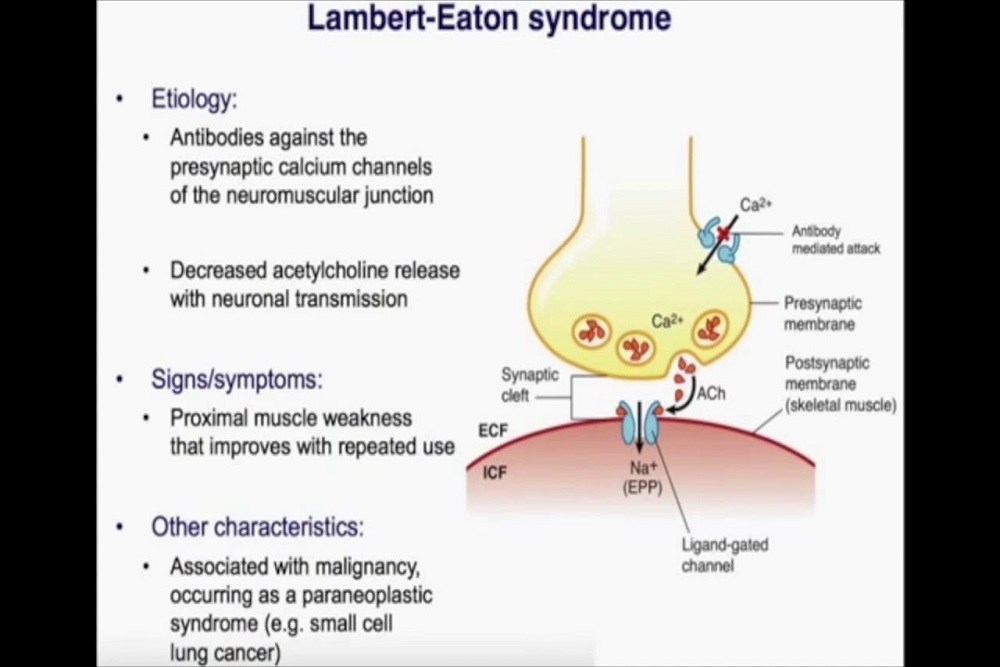
BioTime, Inc. (NYSE American and TASE: BTX), a clinical-stage biotechnology company developing cellular therapies for unmet needs, announced today the issuance of a Notice of Allowance for a patent from the United States Patent and Trademark Office (USPTO) for United States Patent Application No. 15/156,316 for a method of reducing spinal cord injury (SCI)-induced parenchymal cavitation in patients that have suffered an acute spinal cord injury.