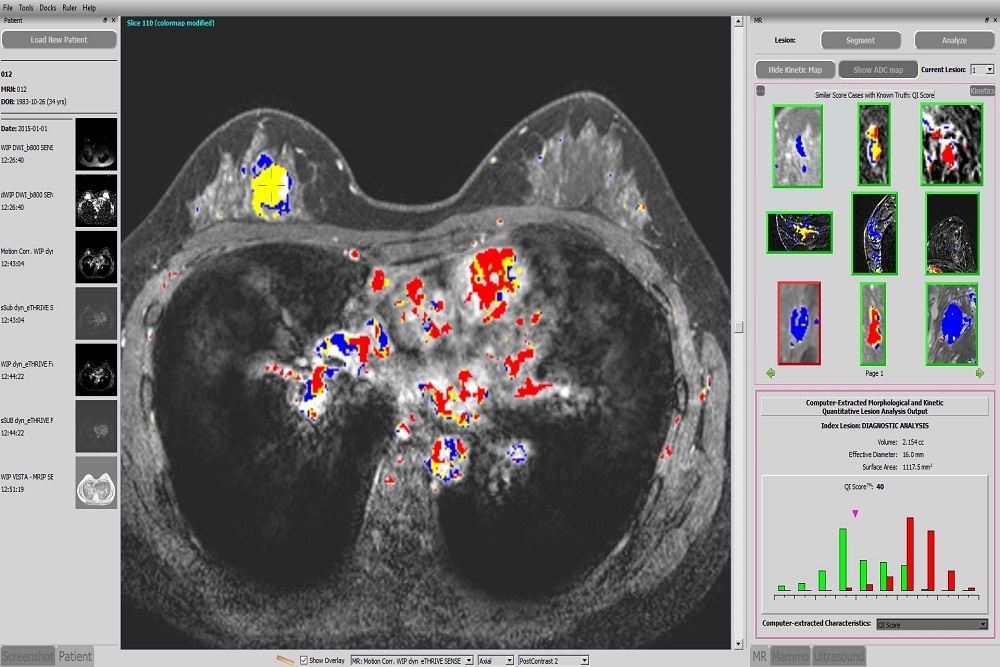
PARAGON BIOSCIENCES: Launches Qlarity Imaging to Advance the First FDA-Cleared Artificial Intelligence Breast Cancer Diagnosis System
Paragon Biosciences LLC — the Chicago-based life science innovator that invests in, builds, and advises bioscience companies — is announcing the launch of its seventh portfolio company, Qlarity Imaging LLC, which was founded to harness the value of artificial intelligence (AI) to improve medical outcomes.