Dynacure issued the following announcement on Aug. 8.
Dynacure, a clinical stage drug development company focused on improving the lives of patients with rare and orphan disorders, today announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation for DYN101, an investigational antisense medicine designed to modulate the expression of dynamin 2 (DNM2) for the treatment of Centronuclear Myopathies (CNM). Dynacure expects to initiate its first in human study, a Phase 1 / 2 study 'Unite-CNM', in the second half of 2019. DYN101 is being developed in collaboration with Ionis Pharmaceuticals, the leader in RNA-targeted drug discovery.
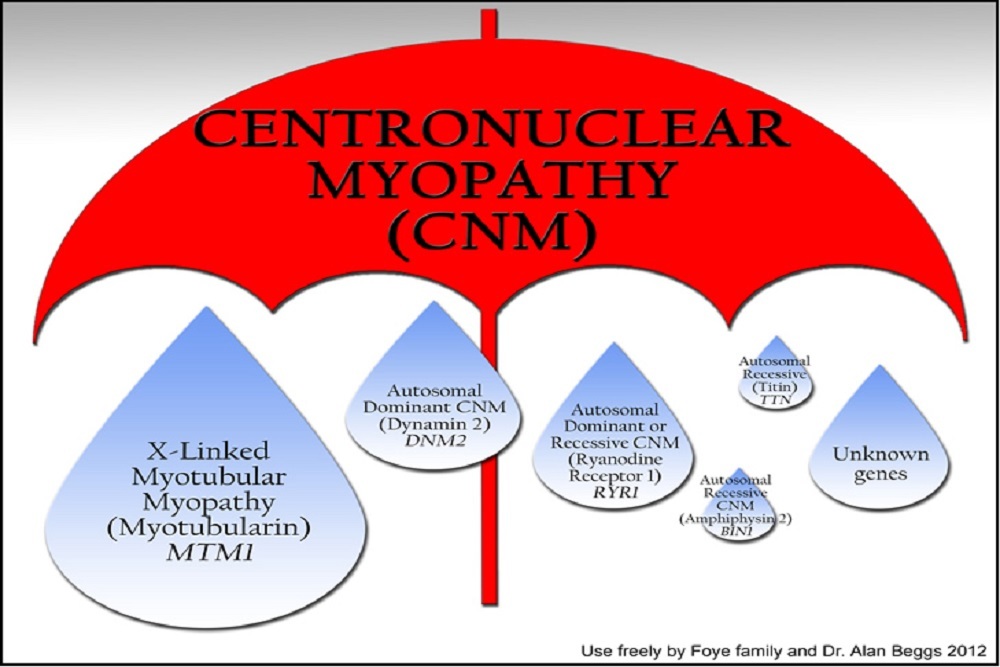
Centronuclear and myotubular myopathies (CNM), are serious, rare, life-threatening disorders that affect skeletal muscles from birth. CNMs derive their name based on the central location of the muscle fiber nucleus, which is an abnormal finding observed in muscle biopsies. The disease is driven by mutations in multiple genes including MTM1, DNM2 and BIN1 and Dynacure scientists have discovered the link between an increase in DNM2 and the direct cause of the disease (Cowling et al 2014 JCI). There are many genetic forms of CNM including X-linked recessive (XLCNM/ Myotubular Myopathy), autosomal dominant (ADCNM), and autosomal recessive (ARCNM), which are all associated with poor prognosis. Centronuclear Myopathies affect between 4,000 and 5, 000 patients in the EU, US, Japan and Australia1.
"Orphan Drug Designation in the US is a critically important regulatory milestone in our global development plan for DYN101 to treat several forms of centronuclear and myotubular myopathies," said Stephane van Rooijen (M.D. MBA), Chief Executive Officer of Dynacure. "The US designation complements our orphan drug designation in the EU and we look forward to enroll in our first-in-human clinical study with DYN101 later this year to treat this devastating rare disease."
The FDA grants Orphan Drug Designation to novel drugs that seek to treat a rare disease or condition and provides 7 years of market exclusivity if approved, plus significant development incentives, including tax credits related to clinical trial expenses, an exemption from the FDA-user fee, and FDA assistance in clinical trial design.
About DYN101 for Centronuclear Myopathies
DYN101, an investigational antisense oligonucleotide using Ionis' proprietary antisense technology, is designed to modulate the expression of dynamin 2 (DNM2) for the treatment of Centronuclear Myopathies. Centronuclear and myotubular myopathies (CNM) are rare congenital myopathies with variable inheritance ranging from X-linked recessive (XLCNM/ Myotubular Myopathy), autosomal dominant (ADCNM), and autosomal recessive (ARCNM), all associated with poor prognosis. Centronuclear Myopathies affect between 4,000 and 5, 000 patients in the EU, US, Japan and Australia1.
Preclinical studies have demonstrated that DYN101 has the potential to be disease modifying in CNM, with compelling preclinical efficacy in treating animal models of XLCNM and ADCNM2,3. Prevention and reversion of the disease was observed with a clear dose-dependent improvement in whole body strength and mice survival.
The development plan for DYN101 was designed to be very broad and it is the only known program being investigated for most CNM populations, XLCNM and ADCNM. In addition to investigating DYN101 for CNM, Dynacure aims to expand its use and explores additional indications where the overexpression of DNM2 is a disease-driving factor.
DYN101 has been granted Orphan Drug designations by the US FDA and EMA.
About the Phase 1 / 2 Study 'Unite-CNM' (DYN101-C101)
'Unite-CNM' (DYN101-C101) is a European multicenter, ascending dose study to evaluate the safety, tolerability, pharmacokinetics and preliminary efficacy of DYN101 in approximately 18 patients greater than 16 years of age with XLCNM or ADCNM. Enrolled patients will have a run-in period or be rolled over from an ongoing natural history study, sponsored by the Institute of Myology in France, which includes 60 subjects that have XLCNM or ADCNM. While the Phase 1 / 2 study will primarily focus on finding an optimal dose of the drug via safety, tolerability and target attainment after 12 weeks of treatment, multiple domains of efficacy will also be assessed in an exploratory analysis, which include muscular function, respiratory function and muscle strength. After completing the Unite-CNM study, Dynacure expects to investigate a potentially registration-directed Phase 2 / 3 study (all age groups) that would include European and US sites.
About Dynacure
Dynacure is a clinical-stage drug development company focused on improving the lives of patients with rare and orphan diseases. The Dynacure team leverages its proven track record in rare disease drug development to build a pipeline of novel drugs. Dynacure is developing DYN101, an investigational antisense medicine designed to modulate the expression of dynamin 2 for the treatment of Centronuclear Myopathies, with Ionis Pharmaceuticals. Dynacure is also building a complementary research portfolio targeting other orphan disorders. The company maintains its headquarters in Strasbourg, France. Dynacure's investors are Andera Partners, Bpifrance, IdInvest, Ionis Pharmaceuticals, Kurma Partners and Pontifax.
Original source can be found here.




