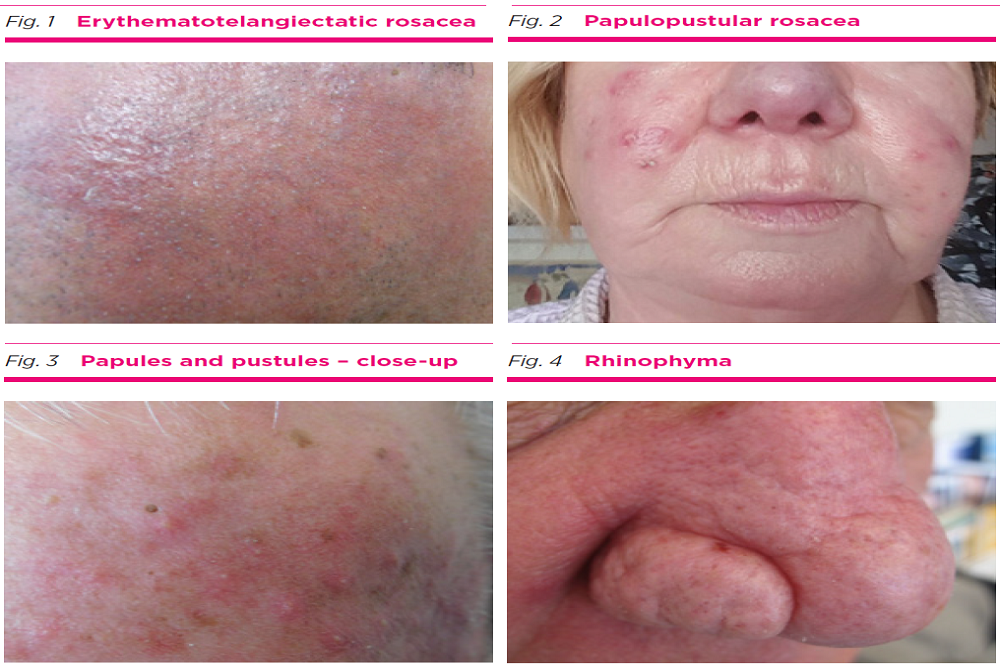
LEO PHARMA: Announces U.S. Food and Drug Administration (FDA) Expanded Regulatory Approvals for Enstilar® Foam and Taclonex® Topical Suspension in Treatment of Plaque Psoriasis
FDA approves Enstilar (calcipotriene and betamethasone dipropionate) Foam, 0.005%/0.064%, for the topical treatment of plaque psoriasis in patients 12 years and older; Agency grants pediatric exclusivity extending U.S. market exclusivity



.jpg)