Foamix Pharmaceuticals issued the following announcement on Aug. 5.
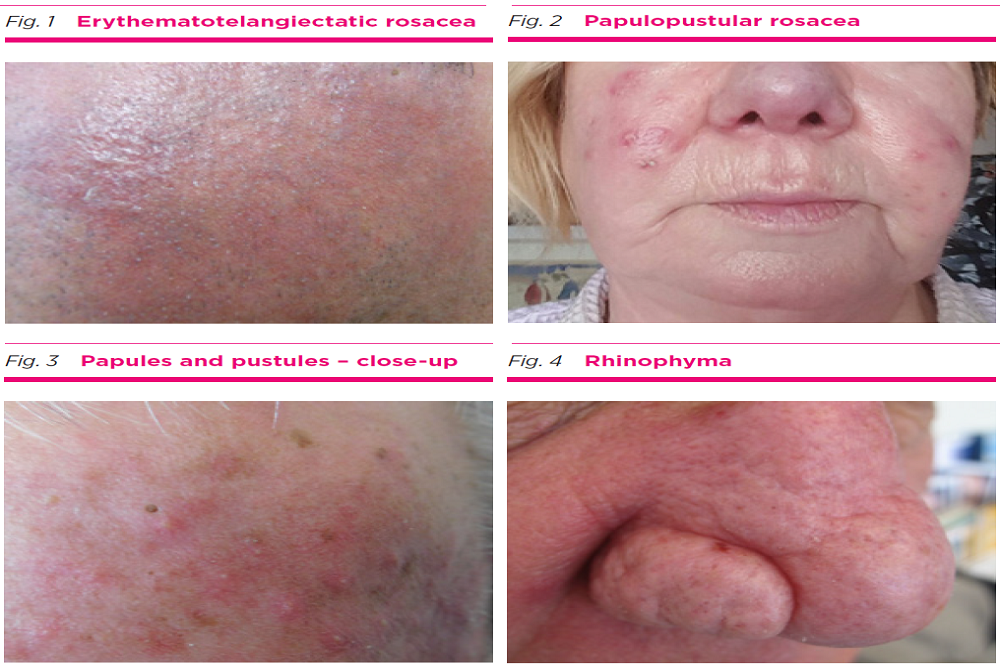
Foamix Pharmaceuticals Ltd. (Nasdaq: FOMX), a clinical stage specialty pharmaceutical company focused on developing and commercializing proprietary topical therapies to address unmet needs in dermatology, today announced that it has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) seeking approval for FMX103 for the treatment of moderate-to-severe papulopustular rosacea in patients 18 years of age and older.
Rosacea is a common skin condition that causes redness and visible blood vessels in the face. It may also produce small, red, pus-filled bumps. These signs and symptoms may flare up for a period of weeks to months and then diminish for a while. Rosacea can be mistaken for acne, an allergic reaction or other skin problems. There are approximately 16 million U.S. rosacea sufferers (source: Aimee Two, MD, et al, JAAD, Volume 72, Issue 5, May 2015), a large percentage of whom (85% 30 years of age and older) suffer multiple comorbidities and experience sensitivity to current treatment options.
"It can be challenging for patients with papulopustular rosacea to find therapies that provide meaningful symptom relief and are also well tolerated when applied to their skin," said David Domzalski, Chief Executive Officer. "Building on the impressive Phase 3 FMX103 topline results announced in November last year, we are excited to have reached this NDA submission milestone earlier than previously anticipated."
The NDA submission is supported by the previously communicated results from two Phase 3 clinical trials, FX2016-11 and FX2016-12. In these trials, FMX103 achieved both co-primary endpoints, demonstrating statistically significant improvements in inflammatory lesion count and Investigator Global Assessment (IGA) treatment success. In both trials, and in the long-term safety extension study FX2016-13, the safety profile of FMX103 was shown to be generally favorable and consistent throughout the clinical development program. The NDA submission also incorporates information on chemistry manufacturing and controls, and data from non-clinical toxicology studies.
"Our goal with developing FMX103 is to provide patients with an efficacious and well-tolerated treatment in a convenient topical foam formulation," stated Iain A. Stuart, Ph.D., Chief Scientific Officer. "This submission for FMX103, which is the second NDA submitted by Foamix within the past 8 months, underscores both the potential of our late stage portfolio in dermatology as well as the strong execution capabilities of our R&D and regulatory teams."
About Foamix Pharmaceuticals
Foamix is a specialty pharmaceutical company focused on the development and commercialization of proprietary, innovative and differentiated topical therapies to treat dermatological diseases. Our leading clinical stage product candidates are FMX101 and FCD105 which are intended for the treatment of moderate-to-severe acne vulgaris and FMX103 which is intended for the treatment of papulopustular rosacea. We continue to pursue research & development of our proprietary, innovative topical delivery technologies for the treatment of various skin conditions. We currently have development and license agreements relating to our technology with various pharmaceutical companies including LEO Pharma A/S and others.
Foamix uses its website (www.Foamix.com) as a channel to distribute information about Foamix and its product candidates from time to time. Foamix may use its website to comply with its disclosure obligations under Regulation FD. Therefore, investors should monitor Foamix's website in addition to following its press releases, filings with the Securities & Exchange Commission, public conference calls, and webcasts.
Original source can be found here.




