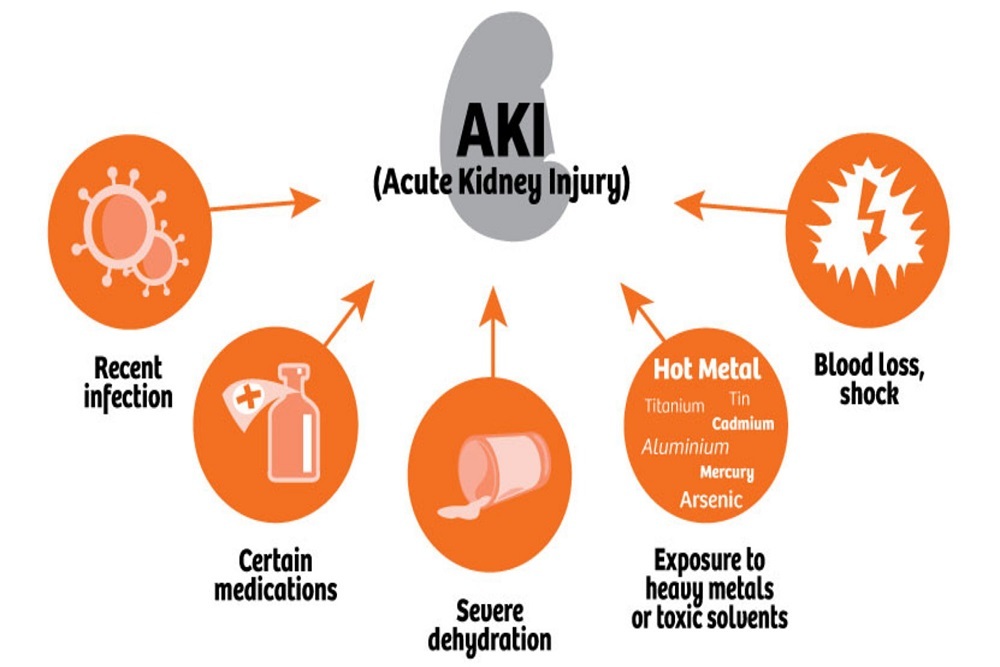
U.S. FDA: Statement on new testing results, including low levels of impurities in ranitidine drugs
Americans deserve to have confidence in the quality of drugs the U.S. Food and Drug Administration regulates – from the prescription medicines they take to the over-the-counter (OTC) products they use in their daily lives.