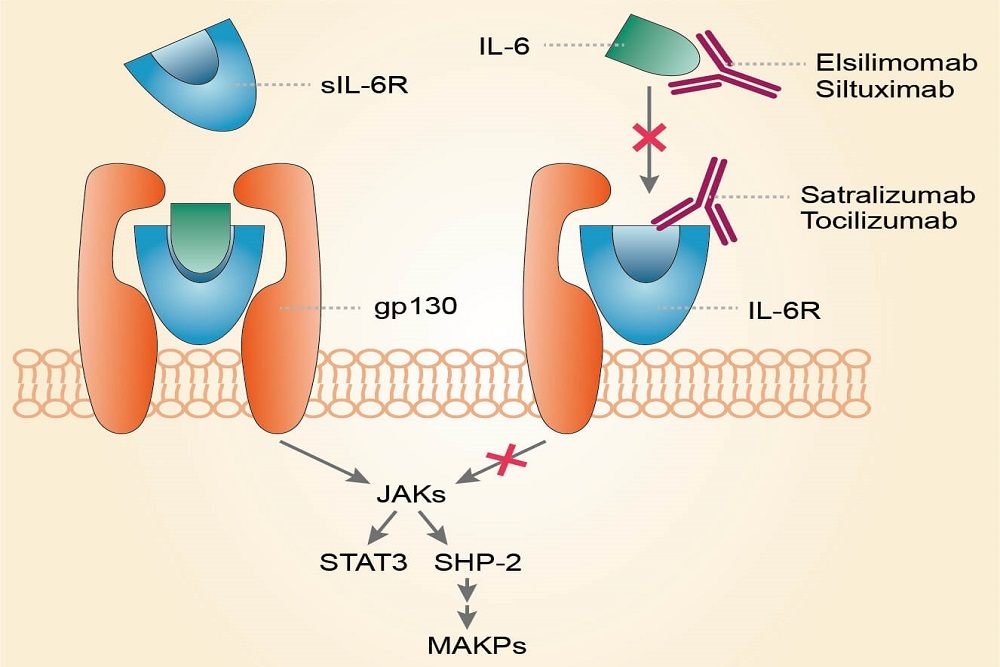
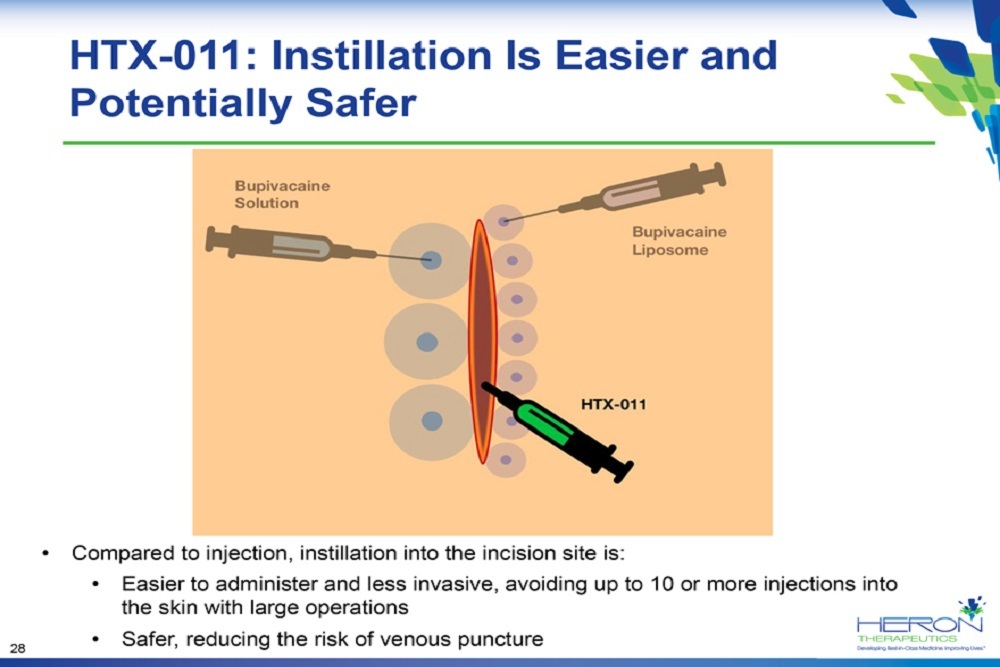
VIZIENT: Comments Support FDA’s Efforts to Expedite Biosimilar Insulin Development
Vizient, Inc. yesterday submitted comments to the Food and Drug Administration (FDA) in response to its draft guidance, “Clinical Immunogenicity Considerations for Biosimilar and Interchangeable Insulin Products.”