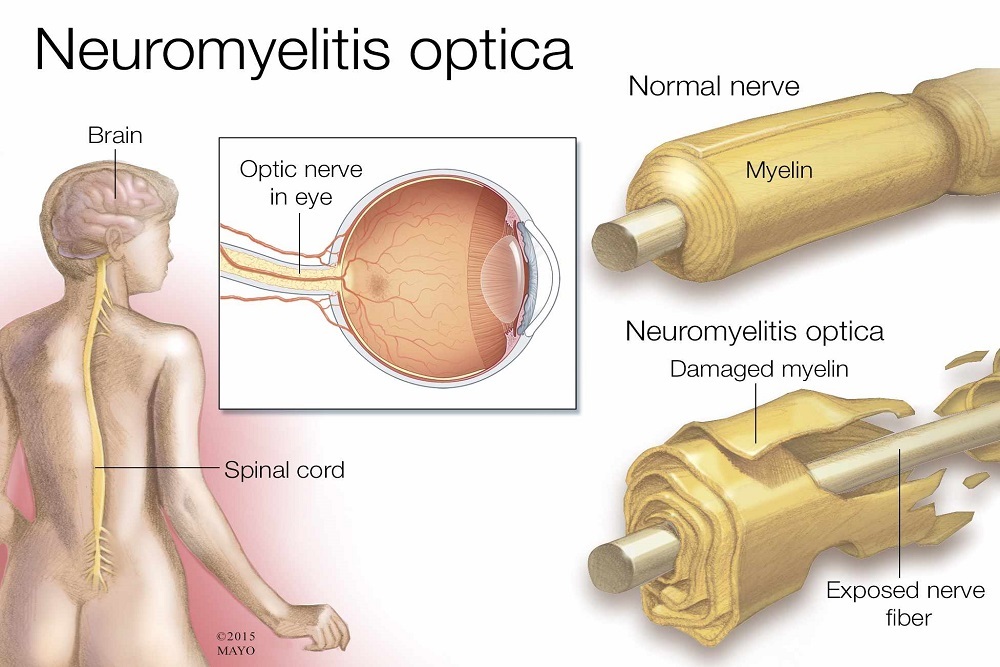
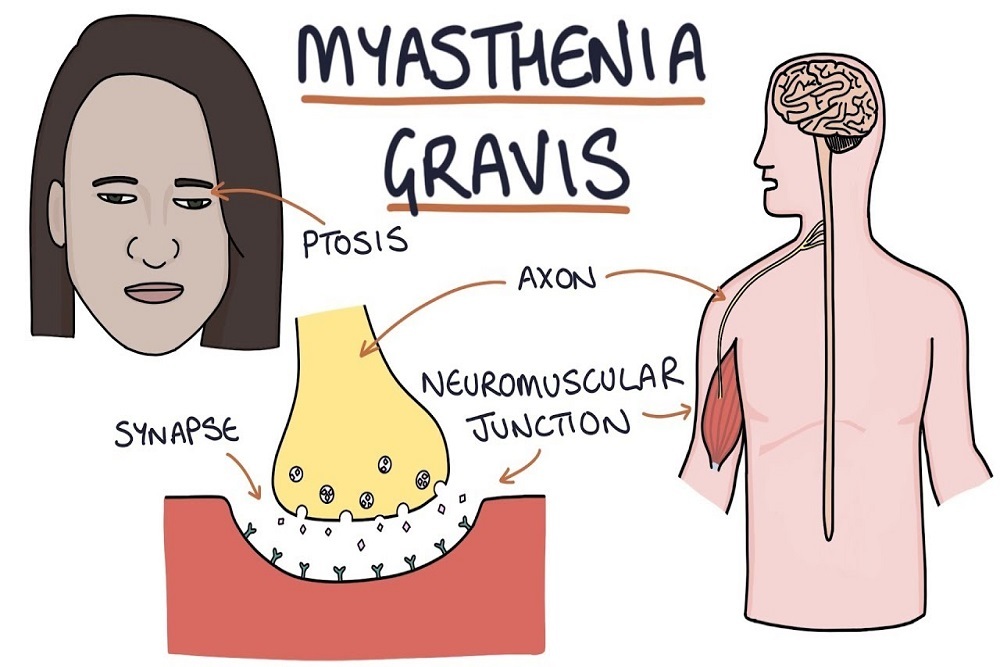
ASTELLAS PHARMA INC.: Seattle Genetics and Astellas Announce U.S. FDA Grants Priority Review for Enfortumab Vedotin Biologics License Application in Locally Advanced or Metastatic Urothelial Cancer
Food & Drug Administration Sets Prescription Drug User Fee Action Date for March 15, 2020