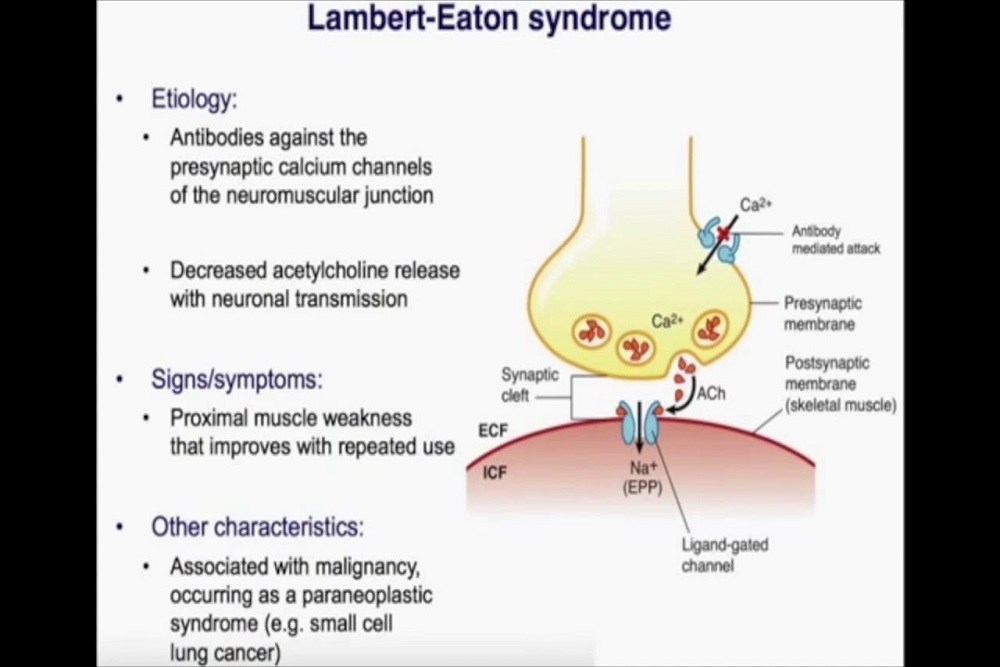
U.S. FOOD AND DRUG ADMINISTRATION: FDA approves first treatment for children with Lambert-Eaton myasthenic syndrome, a rare autoimmune disorder
The U.S. Food and Drug Administration today approved Ruzurgi (amifampridine) tablets for the treatment of Lambert-Eaton myasthenic syndrome (LEMS) in patients 6 to less than 17 years of age.