FDA Update: September medical device recalls
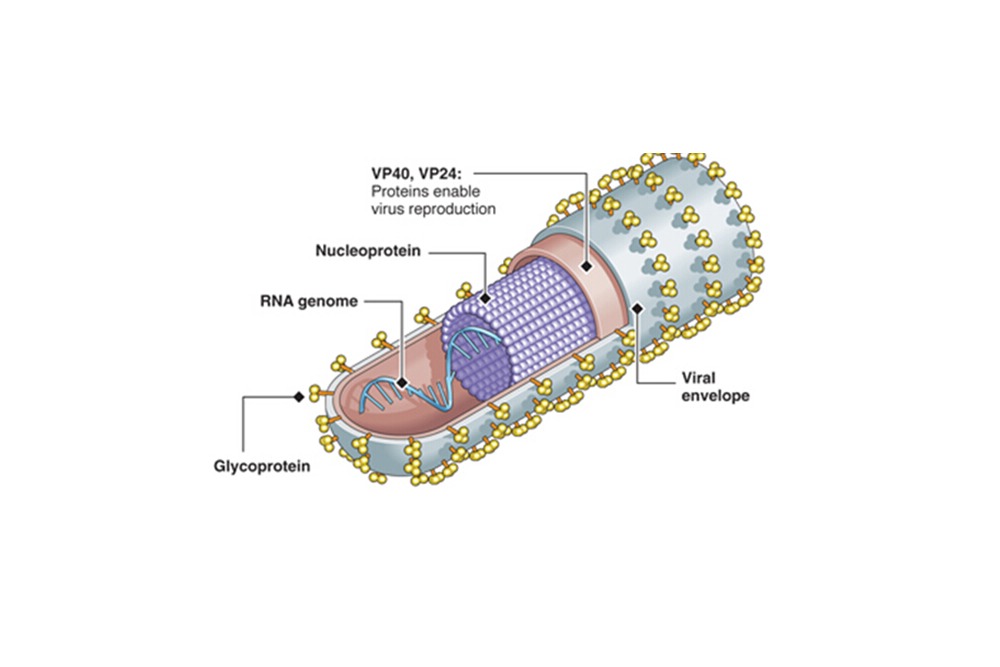
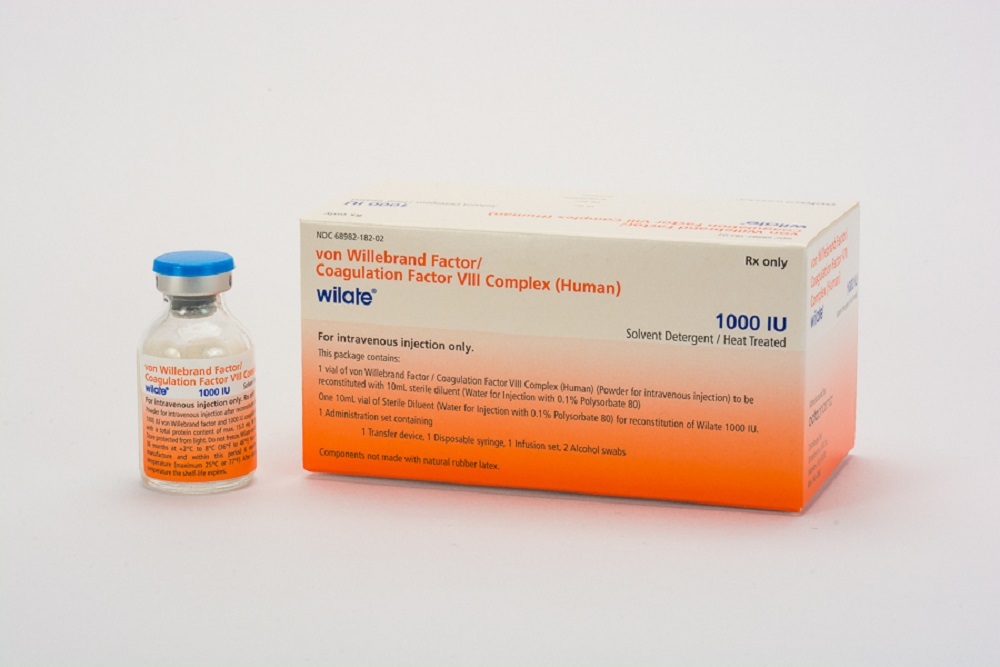
Recalls of medical devices occur when a company sees a need to either take corrective action or remove a potentially hazardous device from the market, according to the U.S. Food and Drug Administration (FDA).