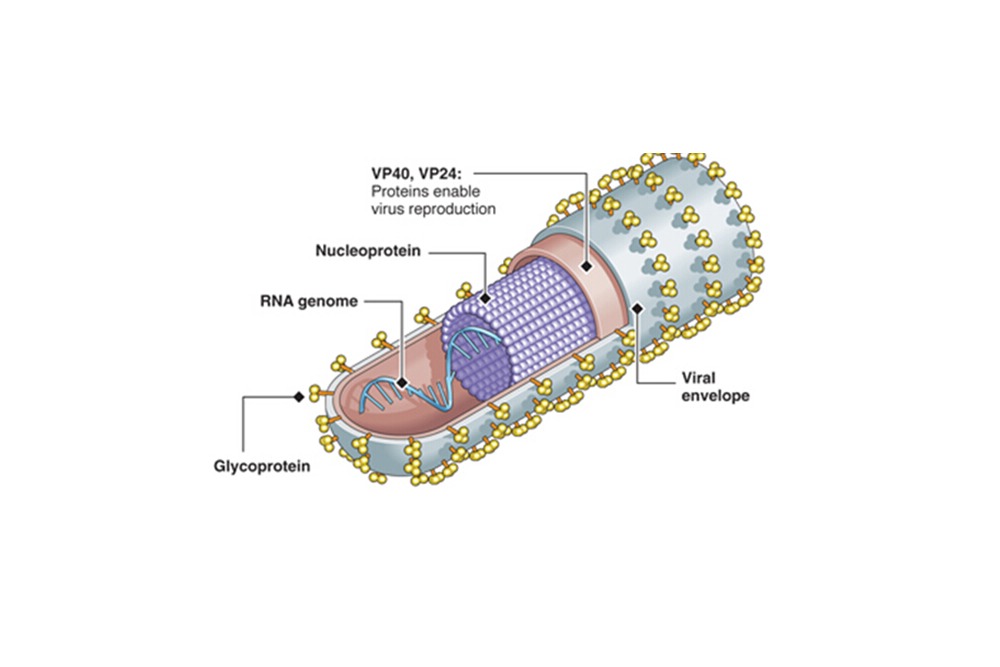
FIRSTKIND LTD: FDA Clears GEKO DEVICE for Use in Non-surgical Patients at Risk for Deep Vein Thrombosis Based on Study Demonstrating a Zero Percent DVT Rate in Patients After a Stroke
Sky Medical Technology furthers presence in 3.5bn1 dollar US market for VTE prevention