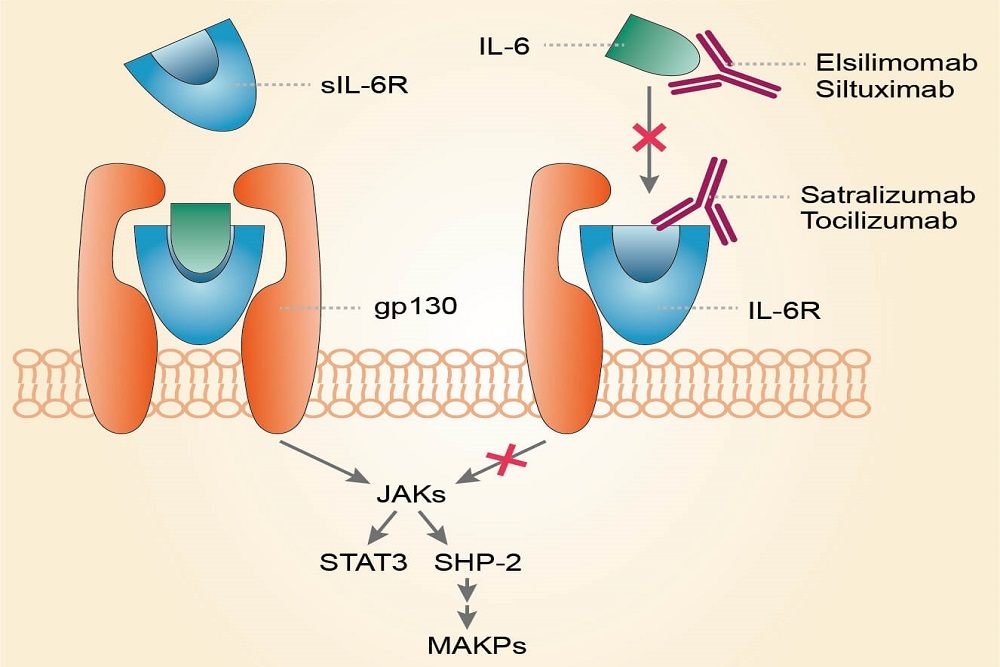
GENETECH: FDA Accepts Genentech’s Biologics License Application for Satralizumab for Neuromyelitis Optica Spectrum Disorder
Satralizumab represents a potential new approach to treating neuromyelitis optica spectrum disorder (NMOSD), a rare, debilitating disease often misdiagnosed as multiple sclerosis (MS)