ACCELERON PHARMA: Receives FDA Orphan Drug Designation for Sotatercept in Pulmonary Arterial Hypertension
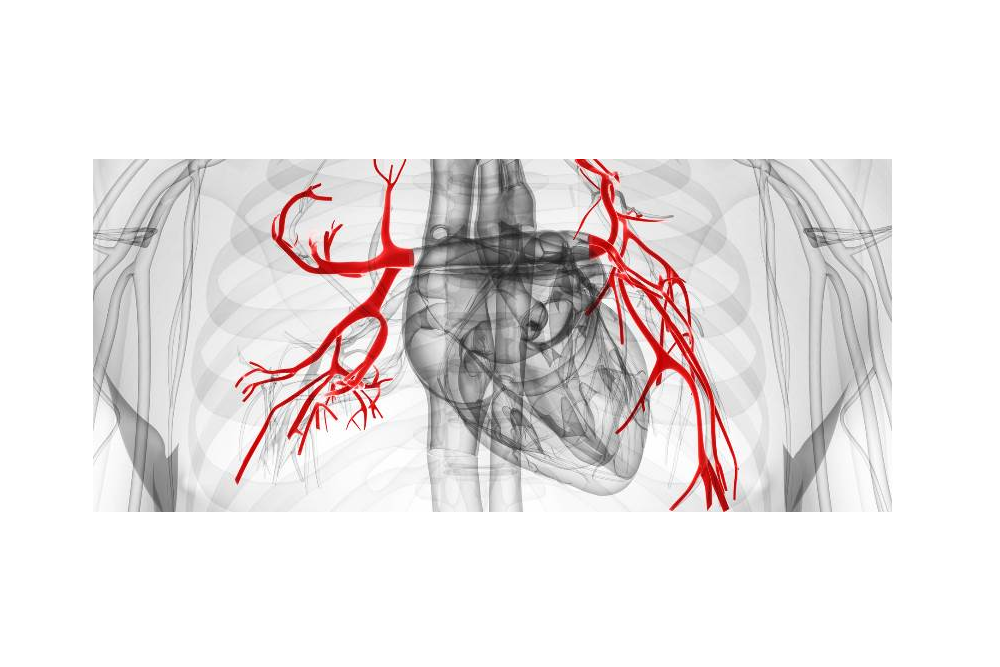
Acceleron Pharma Inc. (NASDAQ:XLRN), a leading biopharmaceutical company in the discovery and development of TGF-beta superfamily therapeutics to treat serious and rare diseases, today announced that the United States Food and Drug Administration (FDA) has granted Orphan Drug designation to sotatercept for the treatment of patients with pulmonary arterial hypertension (PAH).