U.S. FOOD AND DRUG ADMINISTRATION: FDA approves first of its kind device to treat pediatric patients with progressive idiopathic scoliosis
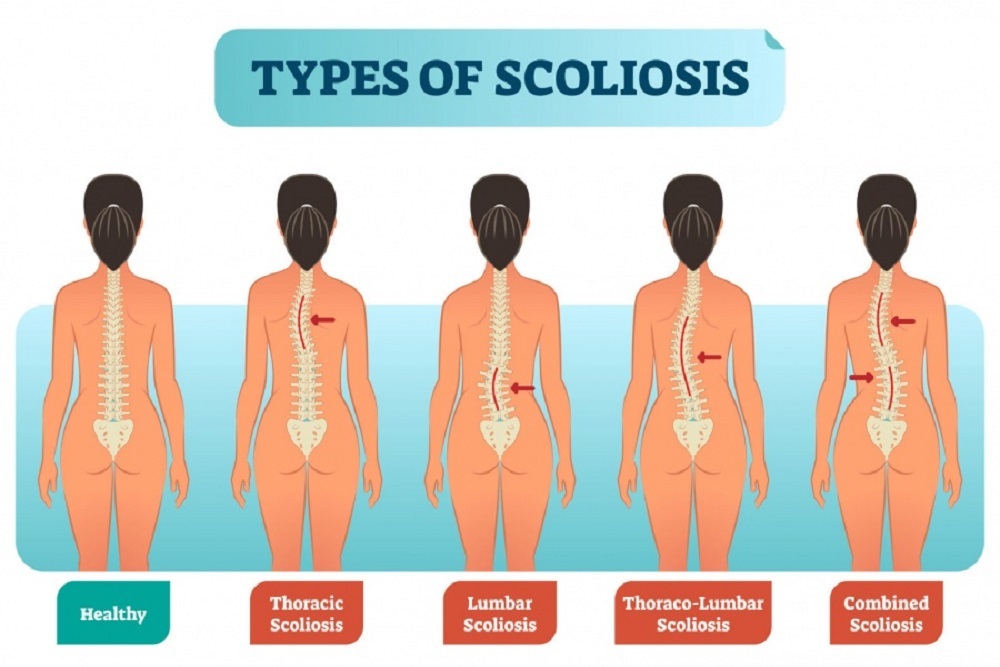
The U.S. Food and Drug Administration today approved the first spinal tether device intended to be used in children and adolescents to correct the most common form of scoliosis, called idiopathic scoliosis, that has not responded to conservative treatment options, such as external bracing.