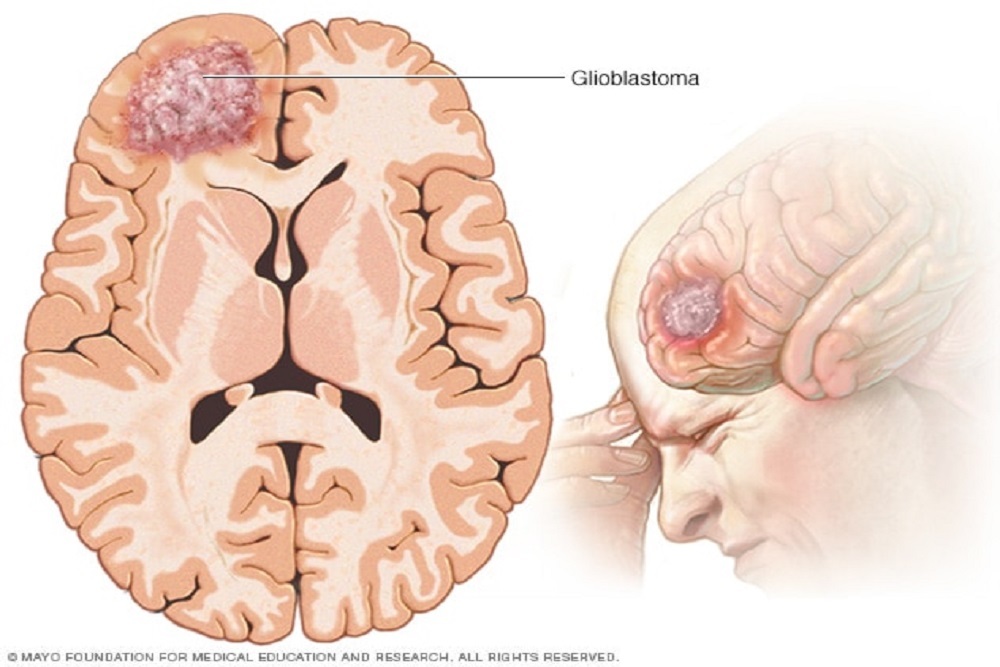
DENOVO BIOPHARMA: Receives FDA's Permission to Proceed with a Biomarker-Guided Phase 2b Clinical Trial with DB102 (Enzastaurin) In First-Line Treatment of Glioblastoma (GBM)
pies, today announced FDA's approval to initiate Denovo's Phase 2b clinical study of DB102 in patients with newly-diagnosed glioblastoma (GBM) in combination with radiation an