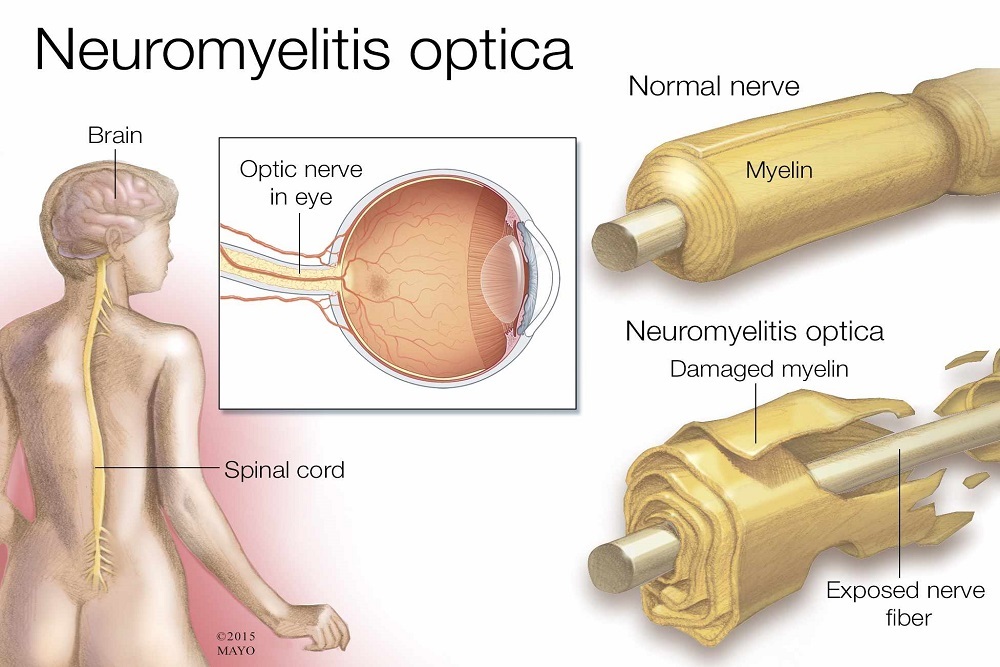
VIELA BIO: Announces Publication in The Lancet of Pivotal Study Results of Inebilizumab in Patients with Neuromyelitis Optica Spectrum Disorder
Viela Bio today announced that peer-reviewed journal, The Lancet, has published results from its pivotal study of inebilizumab in patients with neuromyelitis optica spectrum disorder (NMOSD). NMOSD is a rare, severe, relapsing, neuroinflammatory autoimmune disease that can result in severe muscle weakness and paralysis, loss of vision, respiratory failure and neuropathic pain.