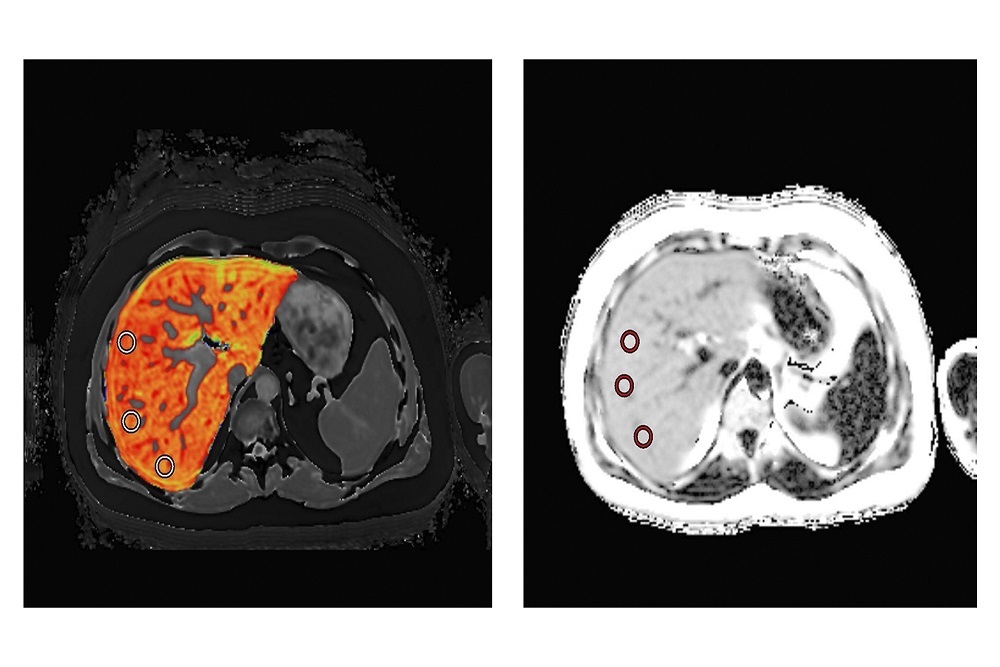
PERSPECTUM DIAGNOSTICS: Announces FDA Grant Award for LiverMultiScan to Help NASH Patients
The Food and Drug Administration (FDA) has awarded Perspectum Inc $250,000 to qualify its proprietary biomarkers for NASH in collaboration with Dr. Naim Alkhouri at the Texas Liver Institute.