Latest News
VESSELON: Acquires FDA-Approved Lipid Microsphere Drug Imagent® For Therapeutic Platform
Vesselon (www.vesselon.com), an oncology therapeutics company, announced the acquisition of an FDA-approved drug Imagent®. Vesselon will use Imagent to create novel therapeutic complexes in four classes of cancer drugs: cytokines, oncolytic viruses, monoclonal antibodies, and nucleic acid constructs.
MCKESSON: TURALIO™ (pexidartinib), FDA Approved Treatment of TGCT, Available at Biologics by McKesson
Exclusive distribution agreement with Daiichi Sankyo, Inc. expands rare disease and complex therapeutic specialty pharmacy needs
BLUEPRINT MEDICINES: Announces FDA Acceptance of New Drug Application for Avapritinib for the Treatment of PDGFRA Exon 18 Mutant GIST and Fourth-Line GIST
FDA grants priority review and sets PDUFA date for February 14, 2020
STOKE THERAPEUTICS: Granted FDA Orphan Drug Designation for STK-001, an Investigational New Treatment for Dravet Syndrome
Stoke Therapeutics, Inc., (Nasdaq: STOK), a biotechnology company pioneering a new way to treat the underlying cause of genetic diseases by precisely upregulating protein expression, today announced that the U.S. Food and Drug Administration (FDA) has granted orphan drug designation to its lead product candidate, STK-001, an investigational new treatment for Dravet syndrome.
U.S. FOOD AND DRUG ADMINISTRATION: Statement on data accuracy issues with recently approved gene therapy
As a public health agency, we believe that it is critical to facilitate the development of innovative safe and effective medical products, like the cellular and gene therapy products that have shown enormous potential to treat previously untreatable diseases.
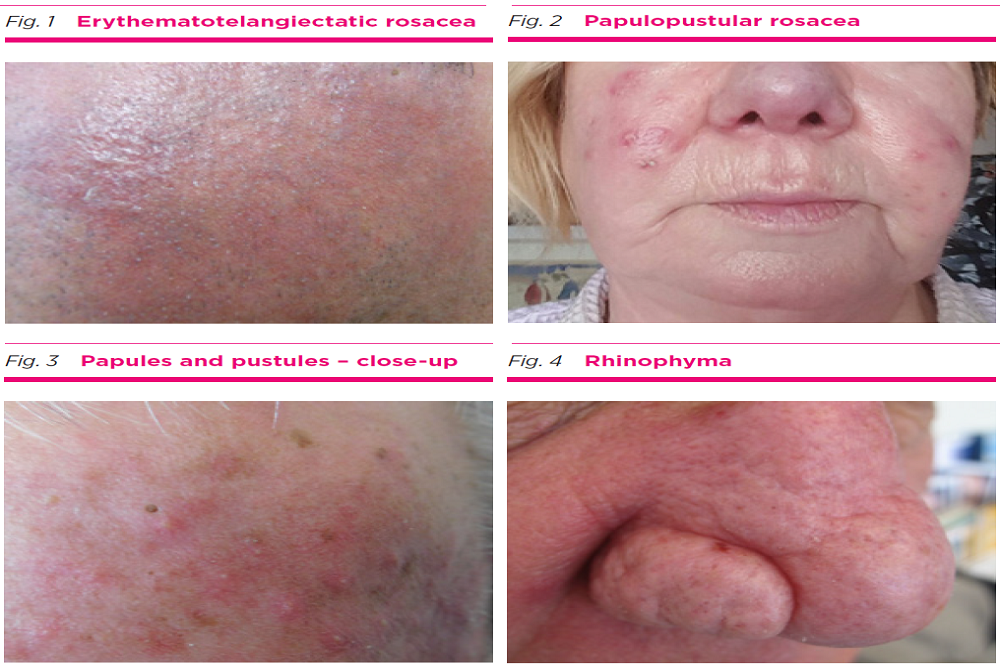
FOAMIX PHARMACEUTICALS: Foamix Submits New Drug Application to U.S. FDA for FMX103 for the Treatment of Moderate-to-Severe Papulopustular Rosacea
Foamix Pharmaceuticals Ltd. (Nasdaq: FOMX), a clinical stage specialty pharmaceutical company focused on developing and commercializing proprietary topical therapies to address unmet needs in dermatology, today announced that it has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) seeking approval for FMX103 for the treatment of moderate-to-severe papulopustular rosacea in patients 18 years of age and older.
ABBVIE: Submits New Drug Application to US FDA for Investigational Elagolix for Management of Heavy Menstrual Bleeding Associated with Uterine Fibroids in Women
New Drug Application (NDA) is supported by data from pivotal Phase 3 studies of nearly 800 patients
Curaleaf receives FDA letter about CBD product marketing
The leading vertically integrated multi-state cannabis operator in the U.S. issued a statement last week about a letter it received from the Food and Drug Administration (FDA) addressing the company's CBD product marketing.
Phase 3 CANDLE study slated for Scynexis treatment of VVC
An agreement between pharmaceutical company Scynexis and the U.S. Food and Drug Administration (FDA) was reached under a special protocol assessment (SPA) on the details of the CANDLE (Conditions Affecting Neurocognitive Development and Learning in Early childhood) study for Oral Ibrexafungerp.
DAIICHI SANKYO: FDA Approves Daiichi Sankyo's TURALIO™ (pexidartinib) for the Treatment of Select Patients with TGCT, a Rare and Debilitating Tumor
TURALIO is the first and only approved therapy for adult patients with symptomatic tenosynovial giant cell tumor (TGCT) associated with severe morbidity or functional limitations and not amenable to improvement with surgery
U.S. FOOD AND DRUG ADMINISTRATION: FDA approves first therapy for rare joint tumor
The U.S. Food and Drug Administration granted approval to Turalio (pexidartinib) capsules for the treatment of adult patients with symptomatic tenosynovial giant cell tumor (TGCT) associated with severe morbidity or functional limitations and not responsive to improvement with surgery.
MCKESSON: NUBEQA® (darolutamide), FDA Approved for Treatment of High-Risk Non-Metastatic Prostate Cancer, Available at Biologics by McKesson
Biologics by McKesson, an independent specialty pharmacy for oncology and other complex therapeutic areas, was selected by Bayer HealthCare as a specialty pharmacy provider for NUBEQA® (darolutamide) for the treatment of non-metastatic castration-resistant prostate cancer (nmCRPC).
LEO PHARMA: Announces U.S. Food and Drug Administration (FDA) Expanded Regulatory Approvals for Enstilar® Foam and Taclonex® Topical Suspension in Treatment of Plaque Psoriasis
FDA approves Enstilar (calcipotriene and betamethasone dipropionate) Foam, 0.005%/0.064%, for the topical treatment of plaque psoriasis in patients 12 years and older; Agency grants pediatric exclusivity extending U.S. market exclusivity
AGILENT TECHNOLOGIES INC.: Agilent Companion Diagnostic Gains Expanded FDA Approval in Esophageal Squamous Cell Carcinoma (ESCC)
PD-L1 IHC 22C3 pharmDx can now be used as an aid to identify ESCC patients
US MEDICAL INNOVATIONS: USMI and JCRI-ABTS Receive FDA Approval to Conduct the First U.S. Clinical Trial Using Cold Atmospheric Plasma for the Treatment of Cancer
US Medical Innovations, LLC (USMI), a Biomedical and Life Science subsidiary of US Patent Innovations, LLC and the Jerome Canady Research Institute for Advanced Biological and Technological Sciences (JCRI-ABTS)™, LLC announced that the U.S. Food and Drug Administration (FDA) has approved the first clinical trial in the U.S. to evaluate Cold Atmospheric Plasma (CAP) Technology for the treatment of cancer.
BAYER: FDA approves Bayer's Nubeqa® (darolutamide), a new treatment for men with non-metastatic castration-resistant prostate cancer
Nubeqa was approved under the FDA's Priority Review designation; approval granted three months ahead of target FDA action date
SUNOVION: Announces Acceptance by the U.S. FDA of the New Drug Application for Dasotraline for the Treatment of Adults with Moderate-to-Severe Binge Eating Disorder
Binge eating disorder (BED) is estimated to affect 4.1 million Americans and may occur at a rate that is three times higher than anorexia and bulimia combined1
MIRUS: Receives FDA Clearance for Lowest Profile Anterior Cervical Plate System
MiRus™ Receives FDA 510(k) Clearance for CYGNUS™ Anterior Cervical Plate
RON SIMON & ASSOCIATES: First Mexican Basil Cyclospora Lawsuit Filed by National Food Safety Law Firm of Ron Simon & Associates as FDA Identifies 132 Victims in Mexican Basil Cyclospora Outbreak
The national food safety law firm of Ron Simon & Associates filed the first lawsuit against Siga Logistics de RL de CV, the Mexican producer and importer of fresh basil linked by health officials to a nationwide cyclospora outbreak.